Research Studies
The Barbara Davis Center for Diabetes (BDC) is currently working on 81 cutting-edge research studies. Here are a few highlights of the advancements in technology and understanding of diabetes in the human body.
Questions about research at the Barbara Davis Center? Then call the Center at 303-724-2323 to learn more or see if you can participate in studies. To find clinical trials going on near you, you can use the search feature here.
Stem Cell Research
Stem cell researchers at the Barbara Davis Center are now able to make insulin-producing beta cells derived from human stem cells on a large-scale. There are only a few labs in the world that are capable of this feat and the hope is to use these cells to treat type 1 diabetes patients.
Several hurdles remain before cell replacement therapy can become a reality. Generated beta cells need an efficient delivery method, as well as effective protection to keep the patient’s immune system from destroying the cells. Dr. Holger A. Russ and his colleagues discovered that more than half of all beta cells die shortly after being transplanted. The lab is now working on strategies to improve the cell survival rate and protect them from autoimmune destruction to eventually use this cell therapy method to cure patients of diabetes.
Dr. Holger A. Russ is currently an Assistant Professor at the Barbara Davis Center for Diabetes. His research focuses on the function of the human pancreatic beta cell, it`s interaction with the immune system, and how autoimmune type 1 diabetes develops. Dr. Russ and his team use this basic knowledge to develop novel strategies for cell therapy approaches to combat diabetes.
Diabetes Technology Research
In the past few years, diabetes technology has made incredible strides and 2018 will show even more technology that will improve life for people with type 1 diabetes. The very first Artificial Pancreas (a system that combines an insulin pump and continuous glucose monitor that aims to keep blood sugars in target range by automatically calculating insulin doses) is now available for the masses, but multiple companies are now in the race with differing features that will improve diabetes care and lessen the burden of diabetes.
Artificial Pancreas Studies
The Barbara Davis Center (BDC) is continuing research on multiple artificial pancreas systems that use a continuous glucose monitor (CGM), which check blood sugar levels every 5 minutes, to stop insulin delivery when a low blood sugar is predicted and to increase insulin delivery when high blood sugars are predicted. These systems have greatly increased time in target range and promise to reduce the burden of care for type 1 diabetes.
The Barbara Davis Center is doing research for multiple Artificial Pancreas systems currently in development. This gives them the unique opportunity to provide feedback to the manufacturers to ensure that patients are receiving the absolute best technology. The research done at the BDC also contributes to the FDA’s decision on whether or not to approve these devices for consumers in the future.
Eventually, there will be multiple Artificial Pancreas systems available commercially, giving patients a choice in the marketplace.
Continuous Glucose Monitors (CGM)
A Continuous Glucose Monitors checks a patients’ sugar level every 5 minutes through a tiny sensor that stays on the body for several days. The device displays graphs to show how quickly blood sugar levels are rising or falling, and alarms when blood sugar levels are too high or low, a feature that is particularly helpful when the patient is sleeping. The device is truly remarkable and even allows a parent to see their child’s blood sugar when the child is at school or even in a different state. This year, research is continuing to make these devices smaller, more accurate, and more user-friendly. As more companies develop systems with unique features, patients will be able to choose the product with features that best fit their needs.
Hispanic/Latino Diabetes Care Program
Dr. Andrea Gerard-Gonzalez joined the faculty of the BDC in 2013 as the Director of the Hispanic/Latino Diabetes Care Program. Her current research focus is to develop successful and cost effective programs to improve the diabetes care delivery and outcomes of Latinos with type 1 diabetes. The BDC serves over 800 Latino/Hispanic pediatric patients diagnosed with T1D and their families. In the US, Hispanic children underutilize diabetes care technology and have higher HbA1c levels than non-Hispanic white children. This is likely related to language barriers, cultural barriers and the limited availability of Spanish-speaking staff and resources. Dr. Gerard-Gonzalez and her team have launched a unique and innovative model of family style shared medical appointments. They are developing brand new educational materials in Spanish and are creating activities that are culturally sensitive and appropriate. The program is focused on reducing costs in high risk patients and building a strong sense of community within Colorado Latino patients with type 1 diabetes.
Basic and Translational Research at the BDC
The BDC Research Division pursues a spectrum of research areas designed to gain insight into the initiation, progression, and pathogenesis (development) of type 1 diabetes. It is the mission of BDC investigators to perform innovative research that will allow us to understand the causes of type 1 diabetes, including disease susceptibilities, genetic and environmental triggers, the autoimmune response, and beta cell dysfunction. An important aspect of our center is to foster closer interactions and collaborations between the immunology and islet biology research programs to synergize our understanding of key events resulting in type 1 diabetes. Furthermore, our research team is working closely with clinical investigators of the BDC to develop intervention and cell replacement therapies to treat and, ultimately, cure type 1 diabetes based on novel research findings.
Investigating the Autoimmunity of Type 1 Diabetes
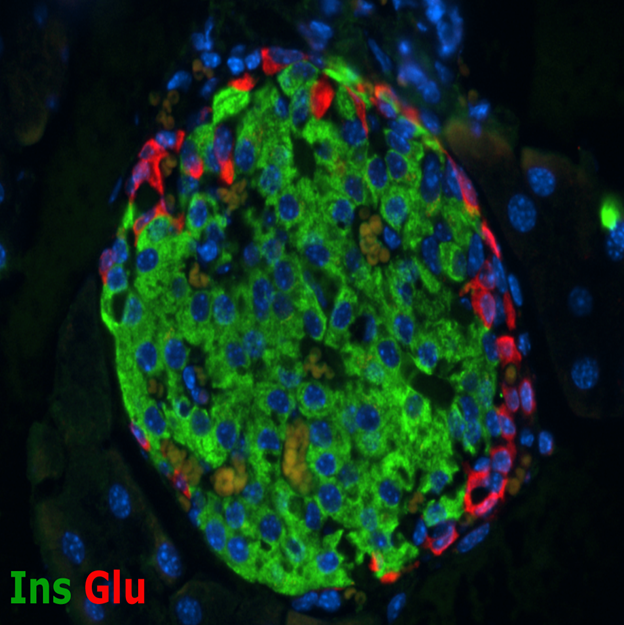
Mouse Islet
A major strength of the BDC has traditionally been associated with research related to the autoimmune response that causes type 1 diabetes. The Center is world renowned for its efforts related to identifying the immune system dysfunctions that trigger islet autoimmunity. This research includes the identification of islet proteins that are mistakenly recognized and targeted as non-self by the immune system, understanding how and why the immune system targets the insulin-producing beta cells, and characterizing the immune response mechanisms that are associated with type 1 diabetes. These studies have led to the development of novel screening tools for disease susceptibility and earlier detection of disease, in addition to innovative intervention therapies to block the immune response after disease onset.
Developing New Sources of Insulin-Producing Cells
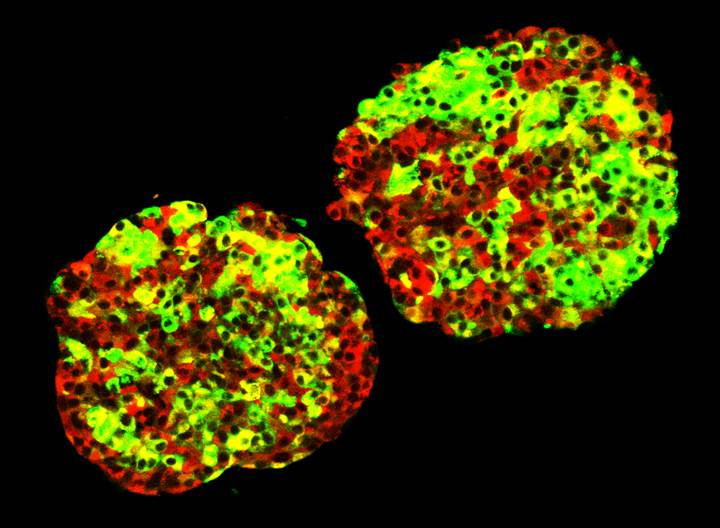
Islet Clusters
The recent addition of new faculty to the Barbara Davis Center is helping to build research focused on islet biology. Although the contribution of beta cell destruction and dysfunction to the etiology of type 1 diabetes has long been appreciated, research focused on understanding how to protect the beta cell during the disease process has been relatively understudied. Furthermore, the success of islet transplantation as a potential treatment for patients suffering from diabetes has highlighted the need for alternative sources of functional islet cells. Several research studies at the BDC are devoted to understanding the mechanisms that govern beta cell development, maturation, replication, and function under normal conditions and in response to stresses associated with autoimmunity.
Another exciting area of research is the generation of infinite numbers of glucose responsive insulin-producing beta cells from patient-derived stem cells. We are currently able to use these cells to model the initiation and progression of human type 1 diabetes in a dish. Ultimately, it is our goal to use these surrogate insulin-producing beta cells to replace the insulin-producing beta cells that are lost during disease progression.
Autoimmunity Screening for Kids (ASK)
The ASK program is a FREE Denver population screening for undiagnosed celiac disease (CD) and preclinical type 1 diabetes (T1D) in children ages 2-17. The ASK program aims to 1) increase awareness around T1D and CD, 2) to prevent diabetic ketoacidosis (DKA) at diagnosis of T1D, and 3) to catch children before onset of disease. The ASK program is currently screening at multiple private pediatric offices in the Denver metropolitan area, Children’s Hospital of Colorado South and Main campus, and at Denver Health’s Eastside clinic. Our plan is to expand throughout the Denver metro area through 2017-2020 in an effort to explore the case for universal screening for T1D and CD in the general population (screening goal of 50-70k). Please visit our website at www.ASKhealth.org or call our ASK hotline at 303.724.1275 for more information and for an update on our current screening locations and times.
Autoantibody/HLA Service Center
The Research Division also offers four different service centers to efficiently facilitate basic and clinical diabetes research. Of particular note, is the Autoantibody/HLA Service Center that is the international reference laboratory for measurement of islet autoantibodies and serves as the national core laboratory for type 1 diabetes TrialNet, The Environmental Determinants of Diabetes in the Young (TEDDY) and the Immune Tolerance Network (ITN) consortia. This laboratory also performs cutting edge research to develop new technologies to predict diabetes in humans and helps to routinely screen all BDC patients for the associated autoimmune conditions.
TEDDY: The Environmental Determinants of Diabetes in the Young
The TEDDY study is currently one of the largest diabetes projects in the United States and the single largest diabetes project in Colorado. This study has been the most comprehensive effort to identify the environmental triggers (viruses, dietary factors, etc.) of type 1 diabetes. Initially, 424,790 newborns from around the world were screened and eventually, 8,677 of the most genetically at-risk newborns were enrolled in the TEDDY study for a 15-year intensive follow up. Of these participants, an astounding 1,374 people are seen at the Barbara Davis Center for Diabetes. The BDC is now analyzing thousands of samples and results in hopes of determining the environmental triggers that cause type 1 diabetes.
DAISY: Diabetes Autoimmunity Study in the Young
DAISY is an observational study that aims to find the cause of type 1 diabetes (T1D). The study, now in its 21st year, identified young children who are at increased genetic risk for the development for T1D and continues to follow them from infancy to adulthood to analyze the development of islet-cell autoimmunity and its subsequent progression to diabetes. DAISY collects data on genetic and environmental exposures for 2,542 children to understand why diabetes is the eventual outcome for some, but not others. Building off of DAISY, other studies have looked into vitamin intake, diet, vaccinations, and genetics. This project has been continuously funded by the National Institute of Health since 1993 and was awarded the distinction of a MERIT project from 2000-2010.
From this study, we have found that:
- 100% of children who are consistently positive for 2 particular islet autoantibodies will develop diabetes in the next 15 years
- Early exposure to cow’s milk does not predict T1D
- Omega-3 fatty acids appear protective
- Routine immunizations and their timing are unrelated to T1D
- The risk is dramatically higher in siblings of children with diabetes
Implementing Annual Depression Screening into Diabetes Clinics
Children with diabetes are at increased risk for depression compared to the general population and this has a significant effect on blood sugar control in adolescents. This is why the BDC is piloting depression screening in the pediatric diabetes clinic to determine the best way to screen and follow-up with patients who are at-risk for depression. Depression in T1D is also associated with increased morbidity due to acute complications, such as increased doctors’ visits and hospitalization and chronic complications, such as cardiovascular disease. By screening and following-up with at-risk patients, the BDC is able to provide complete clinical care for their patients. Routine annual screening for depression is encouraged for all diabetic youths over the age of 10.
High Risk Interdisciplinary Clinic
This program aims to establish continuity of care for high risk patients by creating a team of health care professionals that they see during each visit to better patients’ care and to develop familiarity between the patient and their team. The highest average A1c levels occur during adolescence and young adulthood, often due to psychosocial and behavioral difficulties. Patients with poorly controlled diabetes are more like to have acute and long-term complications. Currently, these patients often see different providers at each visit and are not always able to see ancillary staff due to time or availability, meaning that the underlying issues that are contributing to their poor control are not discovered or addressed. A High Risk Task Force has been developed to work on ways to provide adequate care to high risk patients that are struggling with their diabetes management. This includes creating a new clinical care model with a team of health care professionals to provide continuity of care to this population.
CEDAR: Celiac Disease Autoimmunity Research
This research was based on DAISY study findings and has been funded since 1995. Dr. Marian Rewers and his colleagues have dissected the occurrence of celiac disease in patients with type 1 diabetes, their relatives, and in the general population.
CACTI: Coronary Artery Calcification in Type 1
CACTI has followed 652 adults with type 1 diabetes and 764 of their non-diabetic spouses and friends since 2000 to better define the causes of premature heart disease and other long-term complications in patients with T1D. The study detects and monitors progression of calcification on coronary arteries. This study has discovered a number of novel genetic, metabolic, and inflammatory factors of potential importance to prevent diabetic complications.
TrialNet
Barbara Davis Center investigators are leading the international TrialNet Prevention Studies, which design safe interventions to prevent or slow the progression of type 1 diabetes. The studies look at the development of autoimmunity and its progression in family members of type 1 diabetics and those who have been recently diagnosed. The BDC proposed 2 trials within TrialNet that have been completed and published and have laid the groundwork for all subsequent clinical trials. The studies look at the use of oral insulin, nutrition, metabolic control, prescriptions, among many others.
Inhaled Insulin
The BDC conducts many clinical trials including several in partnership with industry sponsors. Success of the clinical trials program is a combination of patient and family participation in cutting edge research as well as maintaining a qualified and experienced research team to carry out the work required for the trials. One example is a trial of inhaled insulin. The BDC is one of six sites working in the United States to partner with Sanofi in a pediatric trial to learn more about dosing of inhaled insulin in patients under 18 years of age. BDC patients will be the first ones to participate in this exciting cutting-edge research with this new insulin delivery system.
FL3X: Flexible Lifestyles Empowering Change
FL3X is a behavioral intervention for adolescents 13 to 16 years old with T1D who need help with their diabetes care. The intervention uses motivational interviewing and problem solving skills to help patients and their parents improve communication and diabetes care. The Barbara Davis Center collaborates with University of North Carolina and Cincinnati Children’s Hospital on this NIH (National Institutes of Health) and Helmsley Charitable Trust funded study.
PERL: Preventing Early Renal Loss
PERL is a JDRF and NIH funded study to prevent decline in renal function in adults with T1D. The study tests the hypothesis that lowering uric acid with allopurinol, a 10 cent a day generic medication, will slow decline in kidney function. The Barbara Davis Center is one of 15 diabetes centers participating internationally.
CoYoT1 (said: coyote): Colorado Young Adults with Type 1
The BDC is piloting CoYoT1 to help teens and young adults (aged 18 to 25) better adapt to living independently with type 1 diabetes. The study uses Vidyo to conduct secure, virtual visits with their physicians, as well as group clinic meetings with other young T1D adults. The program encourages patients to take charge of their health, preventing unnecessary complications. This program is accessible because it lessens the travel and commitment time required for appointments. Initial feedback has shown that 100% of the participants felt comfortable talking with a doctor online and 95.5% would like to have another online appointment.
Telemedicine
Telemedicine at the BDC is a rapidly growing program that includes a clinical component to provide diabetes care for pediatric patients using secured videoconferencing for virtual visits in partnership with diabetes educators at outpatient office-based sites in Wyoming and western Colorado. The program has increased access to specialized diabetes care and decreased missed work and school time for parents and patients including many with limited ability to travel to the BDC for in person care. The telemedicine program will seek funding to support expansion of the program to serve patients in the region.
Questions about research at the Barbara Davis Center? Then call the Center at 303-724-2323 to learn more or see if you can participate in studies.


