New Predictive Low Glucose Suspend Feature to Launch with Dexcom G6 CGM System Integration
n
n
n
n
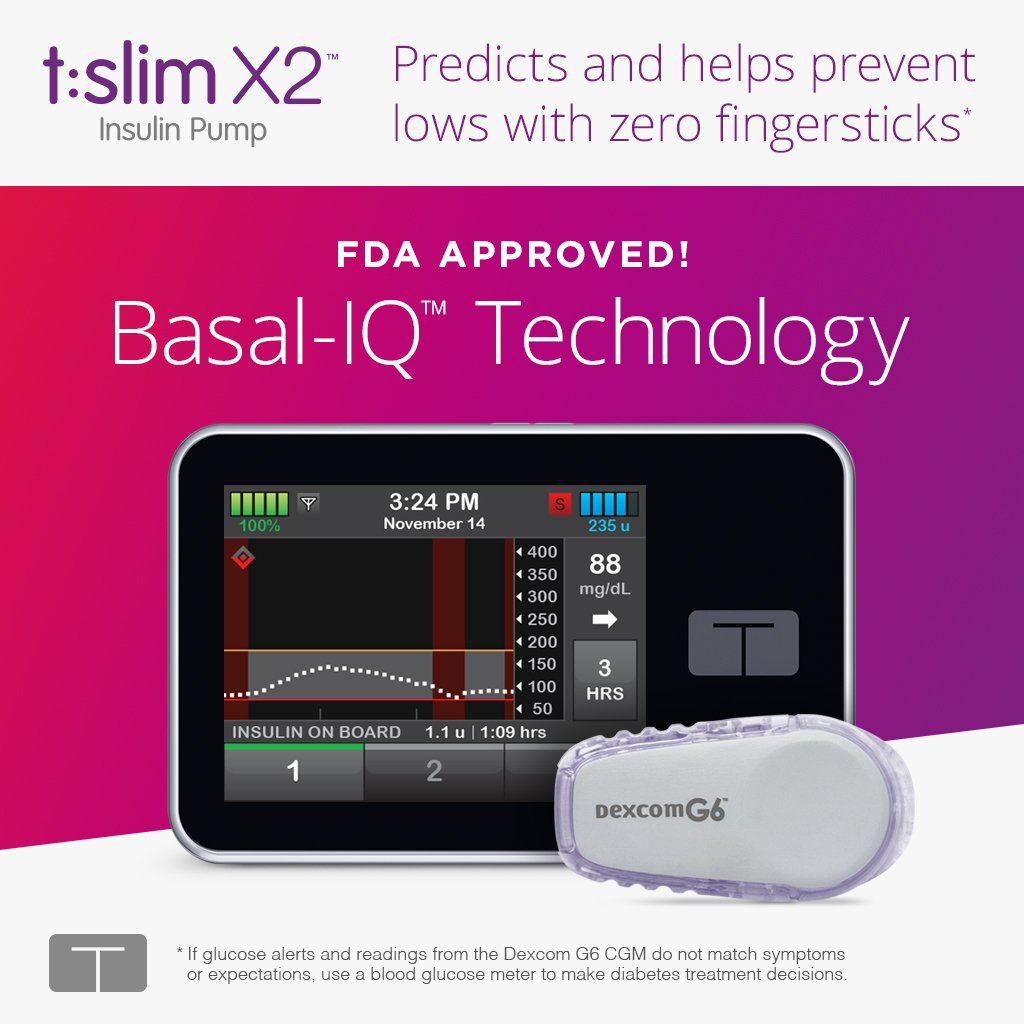 Tandem Diabetes Care®, Inc., a medical device company and manufacturer of the only touchscreen insulin pumps available in the United States, today announced U.S. Food and Drug Administration (FDA) approval of the t:slim X2™ Insulin Pump with Basal-IQ™ technology, a predictive low glucose suspend (PLGS) feature designed to help reduce the frequency and duration of low glucose events (hypoglycemia).nnThis is the first automated insulin delivery system approved for use by children as young as 6 years old, and the first insulin pump designated as compatible with integrated continuous glucose monitoring (iCGM) devices. The Company plans to launch its new product with Dexcom G6® continuous glucose monitoring (CGM) integration, which requires no fingersticks for calibration or diabetes treatment decisions and was the first CGM device to receive the iCGM designation from the FDA earlier this year. nnTandem expects the t:slim X2 Pump with Basal-IQ technology to be available in August 2018, and all in-warranty t:slim X2 users in the United States will have the option to add the new feature free of charge via remote software update.nnTandem’s Basal-IQ algorithm is designed to look 30 minutes into the future to predict where glucose levels are heading. The device suspends insulin delivery when low glucose is predicted, then automatically resumes insulin delivery once glucose levels begin to rise. Use of the t:slim X2 Pump with Basal-IQ technology in the pivotal clinical study demonstrated a 31 percent relative reduction in time spent below 70 mg/dL, with no rebound hyperglycemia compared to a CGM-enabled insulin pump without the feature.nn“Hypoglycemia can be a significant source of fear for people who use insulin and is a major cause of hospitalizations for people with diabetes,” said Dr. Greg Forlenza, Assistant Professor of Pediatrics at the Barbara Davis Center, and one of the principal investigators in the PROLOG (PLGS for Reduction of Low Glucose) trial. “Basal-IQ technology proved very effective in the clinical trial at reducing time spent in hypoglycemia and improving time in range. The system was also simple to learn and use, so the training burden was minimal for the clinics and study participants compared to other automated insulin delivery systems.”nn
Tandem Diabetes Care®, Inc., a medical device company and manufacturer of the only touchscreen insulin pumps available in the United States, today announced U.S. Food and Drug Administration (FDA) approval of the t:slim X2™ Insulin Pump with Basal-IQ™ technology, a predictive low glucose suspend (PLGS) feature designed to help reduce the frequency and duration of low glucose events (hypoglycemia).nnThis is the first automated insulin delivery system approved for use by children as young as 6 years old, and the first insulin pump designated as compatible with integrated continuous glucose monitoring (iCGM) devices. The Company plans to launch its new product with Dexcom G6® continuous glucose monitoring (CGM) integration, which requires no fingersticks for calibration or diabetes treatment decisions and was the first CGM device to receive the iCGM designation from the FDA earlier this year. nnTandem expects the t:slim X2 Pump with Basal-IQ technology to be available in August 2018, and all in-warranty t:slim X2 users in the United States will have the option to add the new feature free of charge via remote software update.nnTandem’s Basal-IQ algorithm is designed to look 30 minutes into the future to predict where glucose levels are heading. The device suspends insulin delivery when low glucose is predicted, then automatically resumes insulin delivery once glucose levels begin to rise. Use of the t:slim X2 Pump with Basal-IQ technology in the pivotal clinical study demonstrated a 31 percent relative reduction in time spent below 70 mg/dL, with no rebound hyperglycemia compared to a CGM-enabled insulin pump without the feature.nn“Hypoglycemia can be a significant source of fear for people who use insulin and is a major cause of hospitalizations for people with diabetes,” said Dr. Greg Forlenza, Assistant Professor of Pediatrics at the Barbara Davis Center, and one of the principal investigators in the PROLOG (PLGS for Reduction of Low Glucose) trial. “Basal-IQ technology proved very effective in the clinical trial at reducing time spent in hypoglycemia and improving time in range. The system was also simple to learn and use, so the training burden was minimal for the clinics and study participants compared to other automated insulin delivery systems.”nnn The Barbara Davis Center for Diabetes was the leading site in acquiring approval from the FDA. Dr. H. Peter Chase has been actively involved with developing the algorithm for the technology for many years.n
The Barbara Davis Center for Diabetes was the leading site in acquiring approval from the FDA. Dr. H. Peter Chase has been actively involved with developing the algorithm for the technology for many years.n
For additional product and safety information, or to begin the order process, visit www.tandemdiabetes.com/tslimX2nor call (877) 801-6901, Monday – Friday between 6am and 5pm Pacific Time
nFree Demo App – Basal-IQ Technology Coming SoonnnTandem’s free t:simulator™ App will be updated in early July to let you experience the touchscreen interface of the t:slim X2 Pump with Basal-IQ technology directly on your mobile device. For more information and to download the app, visit http://www.tandemdiabetes.com/tsimulator.nnFree Software Update for Current t:slim X2 Pump UsersnnAll in-warranty t:slim X2 Pump users in the United States have the option to add the Basal-IQ feature free of charge via a software update using a personal computer. The Basal-IQ feature update will require a user to secure a new prescription from his or her healthcare provider and complete a 45-minute online training module. Internet and computer access are required for pump updates. Tandem expects the Basal-IQ software update to be available for current t:slim X2 Pump users in August 2018. Information about the requirements and update process is available at www.tandemdiabetes.com/X2update.nnBasal-IQ Technology – Clinical OutcomesnnIn February 2018, the Company reported results from the six-week PROLOG (PLGS for Reduction of Low Glucose) pivotal trial, a randomized crossover study comparing two three-week periods of at-home insulin pump use, one period using the t:slim X2 Pump with Basal-IQ technology, and another period using a standard CGM-integrated t:slim X2 Pump without automated insulin suspension. The study included 103 participants with type 1 diabetes age 6 to 72 at four research centers across the United States. Use of the system with Basal-IQ technology reduced the number of sensor glucose readings below 70 mg/dL by 31 percent compared to the control period using a standard CGM-integrated t:slim X2 Pump without automated insulin suspension. Importantly, this marked reduction of time spent in low glucose was accomplished without any increase in the rate of hyperglycemia and participants overwhelmingly described the system as simple to learn and use.nnInsulin Pump Use and DiabetesnnDiabetes is a chronic, life-threatening disease that affects more than 29 million people in the United States, or nearly 1 in 10 Americans. Tandem estimates that more than three million people in the United States require daily administration of insulin and are candidates for pump therapy. More than 425,000 Americans with type 1 diabetes use an insulin pump, or approximately 27 percent of the type 1 diabetes population. In addition, approximately 125,000 Americans with type 2 diabetes use an insulin pump, a small fraction of the type 2 diabetes population.nnAbout Tandem Diabetes Care, Inc.nnTandem Diabetes Care, Inc. (www.tandemdiabetes.com) is a medical device company dedicated to improving the lives of people with diabetes through relentless innovation and revolutionary customer experience. The Company takes an innovative, user-centric approach to the design, development and commercialization of products for people with diabetes who use insulin. Tandem manufactures and sells the t:slim X2™ Insulin Pump with Basal-IQ™ technology. The t:slim X2 Pump is capable of remote feature updates using a personal computer, and is the only automated insulin delivery device approved for children as young as 6 years old. Tandem is based in San Diego, California.nnTandem Diabetes Care is a registered trademark, and t:slim X2, Basal-IQ and t:simulator are trademarks of Tandem Diabetes Care, Inc. Dexcom and Dexcom G6 are registered trademarks of Dexcom, Inc. All other trademarks are the property of their respective owners.nn
n
n
n

